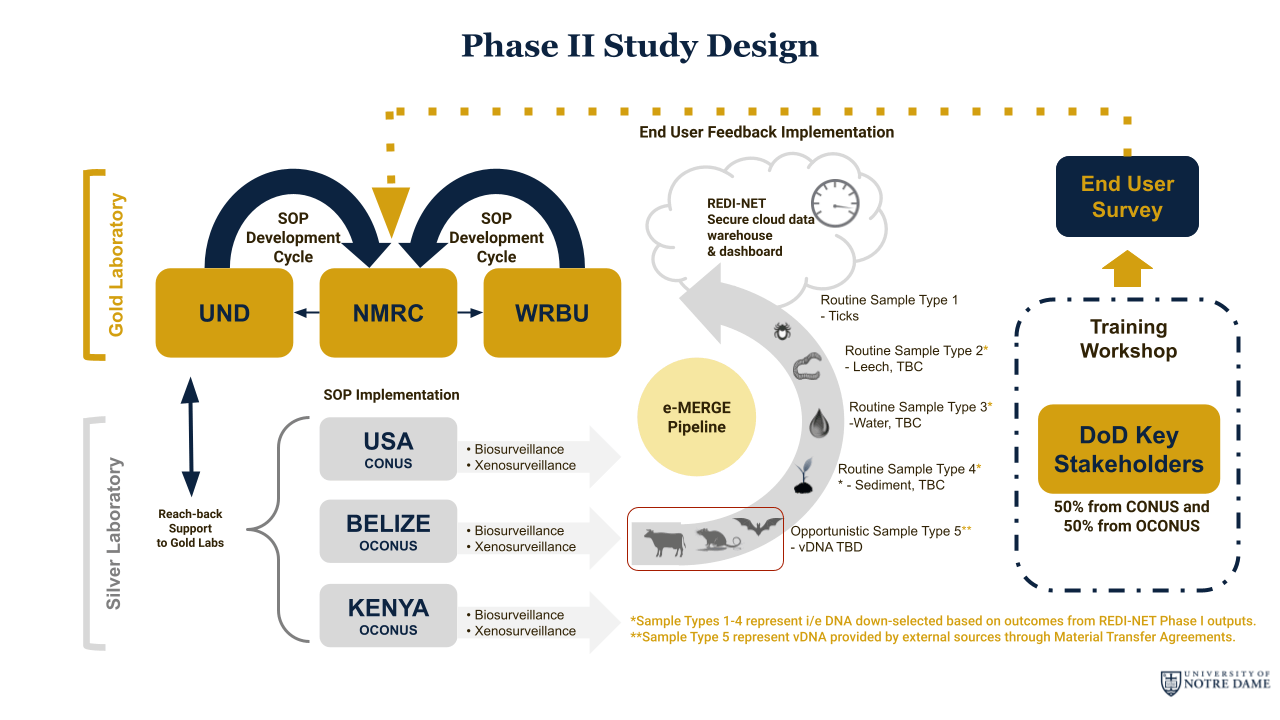
Phase II
During phase II, the REDI-NET program generated evidence through two levels of laboratories to evaluate three hypotheses that informed improvement in forecasting potential risk of exposure for local communities. Gold labs include the University of Notre Dame, the Naval Medical Research Center, and the Walter Reed Biosystematics Unit. Silver labs consist of the Belize Vector and Ecology Center (Belize), the Navy Entomology Center of Excellence (USA), and the Mpala Research Centre (Kenya). In addition, the inclusion of external partnerships has enabled the expansion of the pathogen portfolio through opportunistic sampling.
Hypotheses evaluated during phase II:
- Expansion of pathogen portfolio to include vertebrate DNA
- Validation of the e-MERGE pipeline
- Knowledge transfer to key CONUS and OCONUS stakeholders
Central to the REDI-NET Phase II goals was the expansion of the e-MERGE pipeline developed in Phase I. The fully flexible, scalable, and expandable electronically merged (e-MERGE) remote pathogen-detection pipeline made the integration of Phase II-generated data seamless, including data from other sample types. Reach-back CONUS Gold labs were able to access and support the cross-seasonal environmental pathogen monitoring of the forward-facing OCONUS Silver labs in real time. This core functionality, facilitated by stringent and robust SOPs, ensured a unified data collection, processing, and sharing strategy from remote global theatres that facilitated rapid, automated downstream analyses of field-generated Next-Generation Sequencing (NGS) data and instantaneous upload of accurate, verified results to the REDI-NET project alert dashboard. The established REDI-NET e-MERGE pipeline developed in Phase I served as the platform for enhancement in Phase II for timely detection and mitigation of potential zoonotic pathogen spillover events in remote theatres.
Phase II Study Design